CareStart™ COVID-19 Antigen Rapid Test
The CareStart™ COVID-19 Antigen Test is an FDA EUA point-of-care (POC) designated test with a 10 minute processing time that allows rapid and effective screening of COVID-19 infection without any special equipment needed.
This lateral flow assay test is easy to administer in a POC setting by a nasopharyngeal (NP) swab by non-laboratory medical professionals and allows for clear, visual interpretation of results onsite, in under 10 minutes. This test manufactured by Access Bio has been authorized by the FDA under an Emergency Use Authorization (EUA). For nearly 20 years, Access Bio has been a global leader in engineering and manufacturing superior-quality diagnostic testing for infectious diseases.
Product Features
- Lateral flow assay
- Rapid results in 10 minutes
- Minimally invasive specimen collection (nasopharyngeal)
- Designed for use in point-of-care settings
- No special equipment needed
- Detect SARS-CoV-2 nucleocapsid protein antigen
- Identify acute infection with high sensitivity and 100% specificity
- Manufactured in the USA
Procedure & Results Interpretation
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay for the detection of extracted nucleocapsid protein antigens specific to SARS-CoV-2 in swab specimens directly collected from individuals who are suspected of COVID-19 by their healthcare providers.
Procedure
Peel off aluminum foil seal and rotate the swab inside the extraction vial vigorously at least 5 times.

Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Properly discard the swab.

Close the vial by pushing the cap firmly onto the vial and mix thoroughly by flicking the bottom of the tube.

Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow three (3) drops of sample to fall into the sample well.

Results Interpretation

Read the result at 10 minutes. The test result should not be read after 15 minutes.


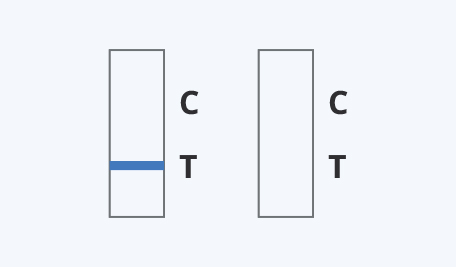
The detection of SARS-CoV-2 nucleocapsid antigen is dependent upon proper specimen collection, handling, storage, and preparation. Failure to observe proper procedures in any one of these steps can lead to incorrect results.
