FaStep® COVID-19 IgG/IgM Antibody Rapid Test
The Assure FaStep® COVID-19 IgG/IgM Antibody Rapid Test is an FDA EUA point-of-care (POC) designated test device with a 15 minute processing time that allows rapid and effective qualitative detection and differentiation of IgM and IgG antibodies (IgM is the first antibody produced in response to an infection; IgG is produced in a delayed response to an infection and can indicate a prior infection or vaccination) to SARS-CoV-2 via fingerstick whole blood without any additional special equipment needed.
This rapid lateral flow chromatographic immunoassay test is easily administered in a POC setting by fingerstick (sterile safety lancet included in kit) whole blood as well as venous whole blood (sodium EDTA), serum, and plasma (sodium EDTA) and allows for clear, visual interpretation of results onsite, in 15 minutes. This test manufactured by Assure Tech has been authorized by the FDA under an Emergency Use Authorization (EUA).

Product Features
- Lateral flow assay
- Rapid results in 15 minutes
- Minimally invasive specimen collection (fingerstick)
- Authorized for use in point-of-care settings
- No special equipment needed
- Detect and differentiate presence of SARS-CoV-2 antibodies IgG/IgM
- Combined Sensitivity: 100% (CI: 88.7%; 100%)
- Combined Specificity: 98.8% (CI: 93.3%; 98.8%)
Procedure & Results Interpretation
The Assure COVID-19 IgG/IgM Rapid Test Device is a rapid lateral flow chromatographic immunoassay intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The Assure COVID-19 IgG/IgM Rapid Test Device should not be used to diagnose acute SARS-CoV-2 infection.
Procedure
(For Fingerstick Whole Blood)Before you begin:
- Refer to the package insert for more information.
- Read through the entire quick reference instructions before beginning a test.
- Bring test components to room temperature before starting.
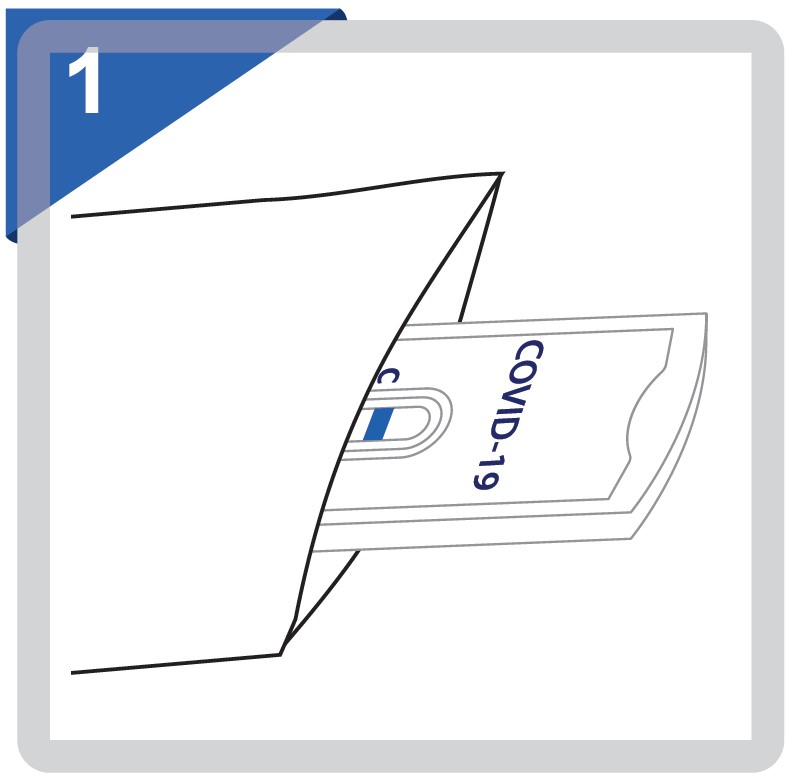
Move the test cassette from the sealed pouch and use it as soon as possible.
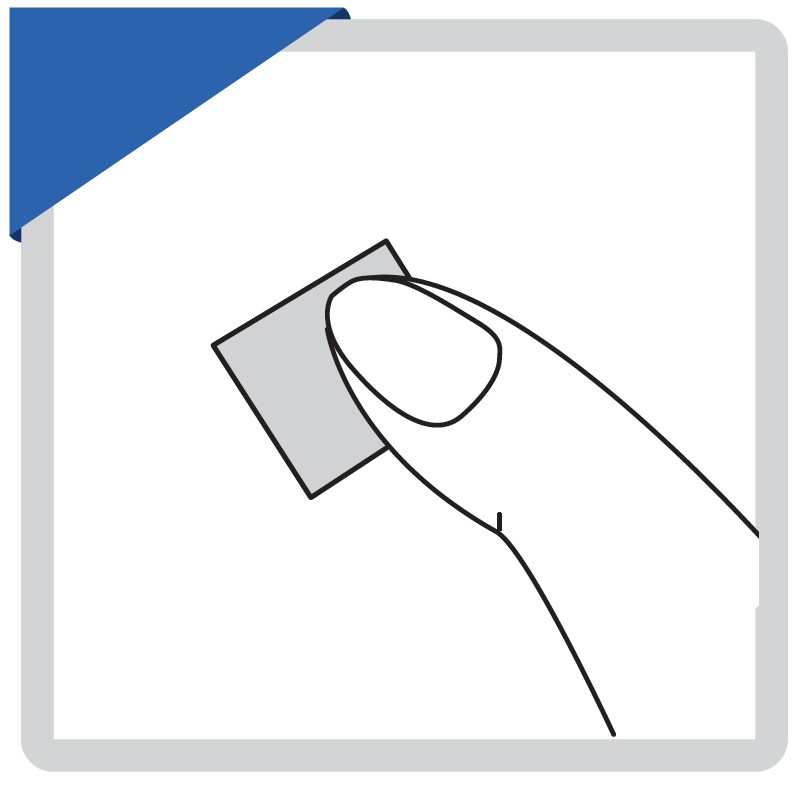
Clean the puncture site with the alcohol prep pad provided.
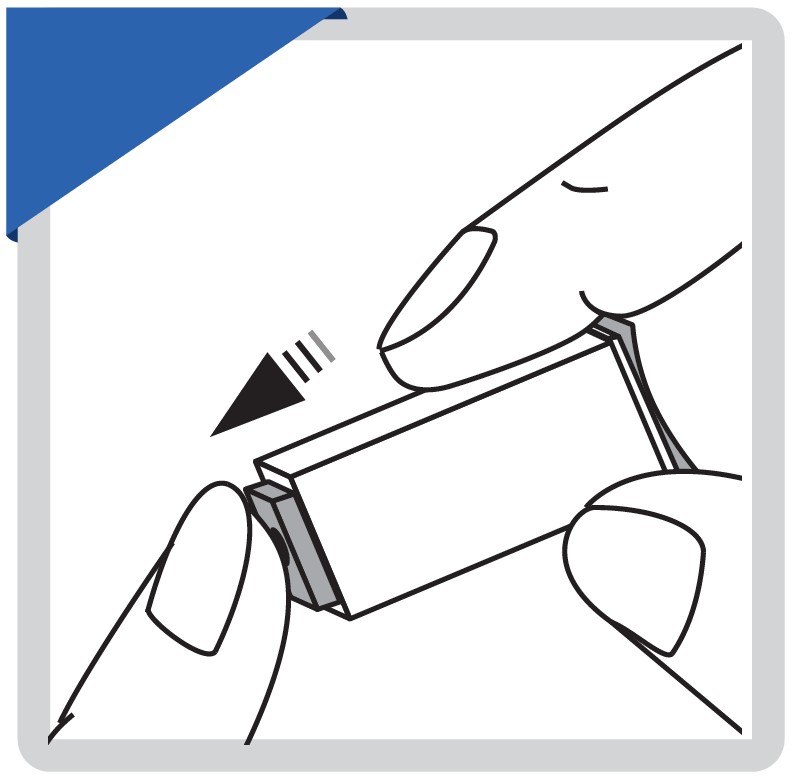
Remove the cap. Push the safety lancet firmly against the puncture site.
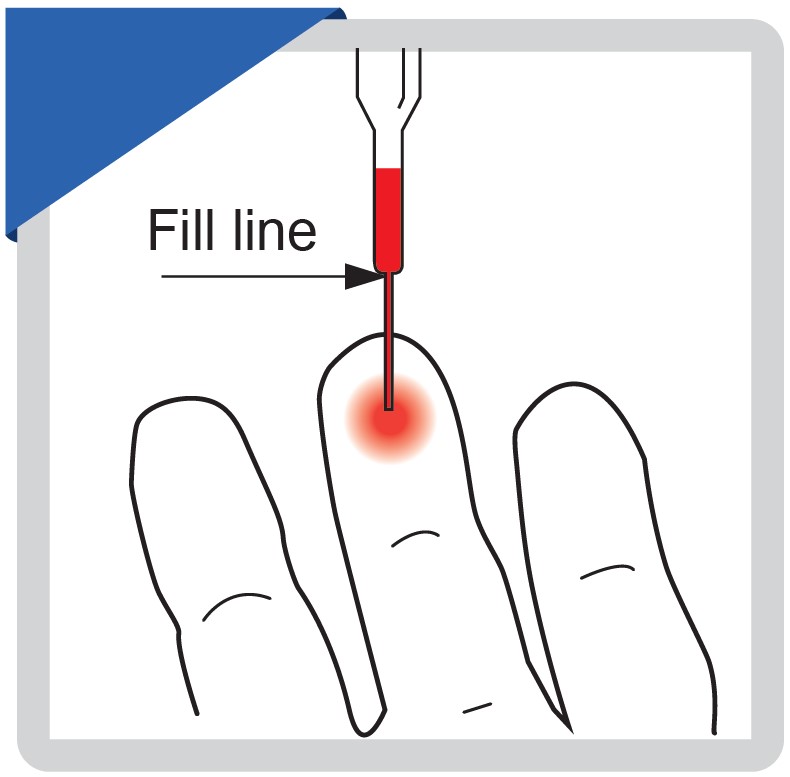
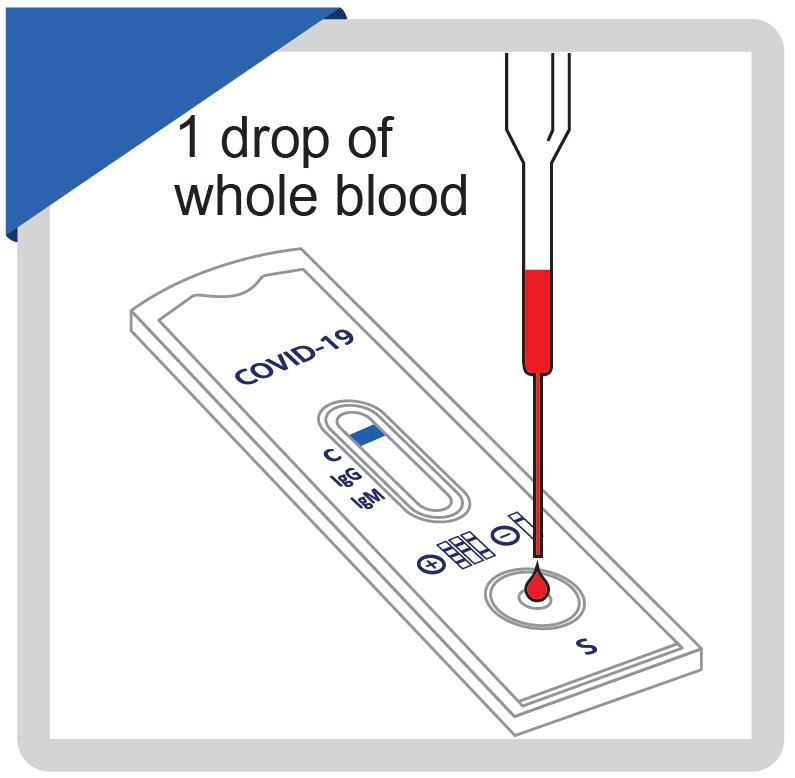
Draw the specimen above the fill line.
Transfer one drop of specimen into the specimen well. Adding more or less than one drop of specimen may lead to erroneous results.
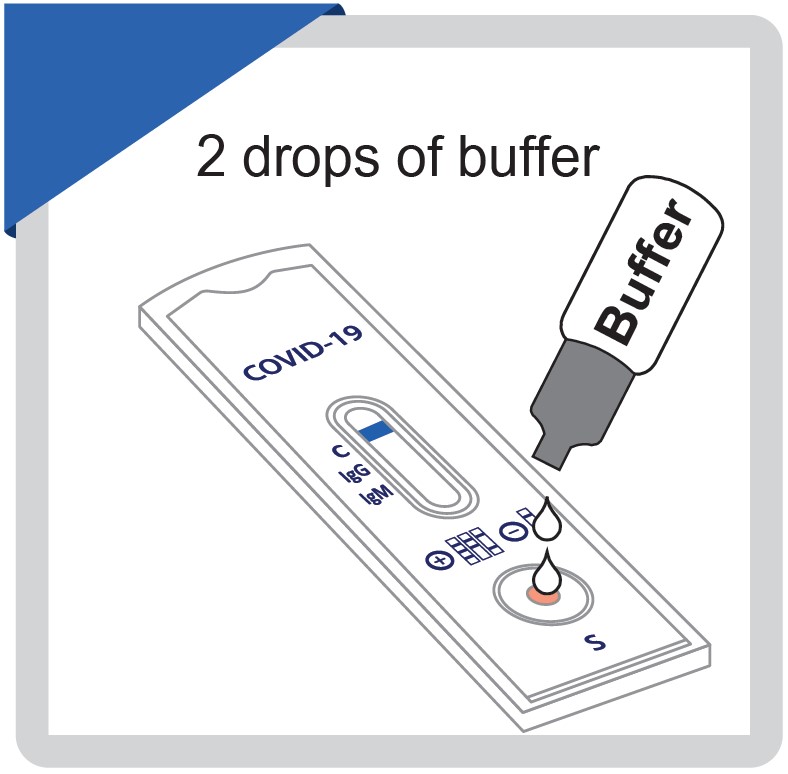
Add two drops of buffer and start the timer. Adding one drop of buffer or more than 4 drops of buffer may lead to erroneous results . Wait for the blue line to change to a red line.
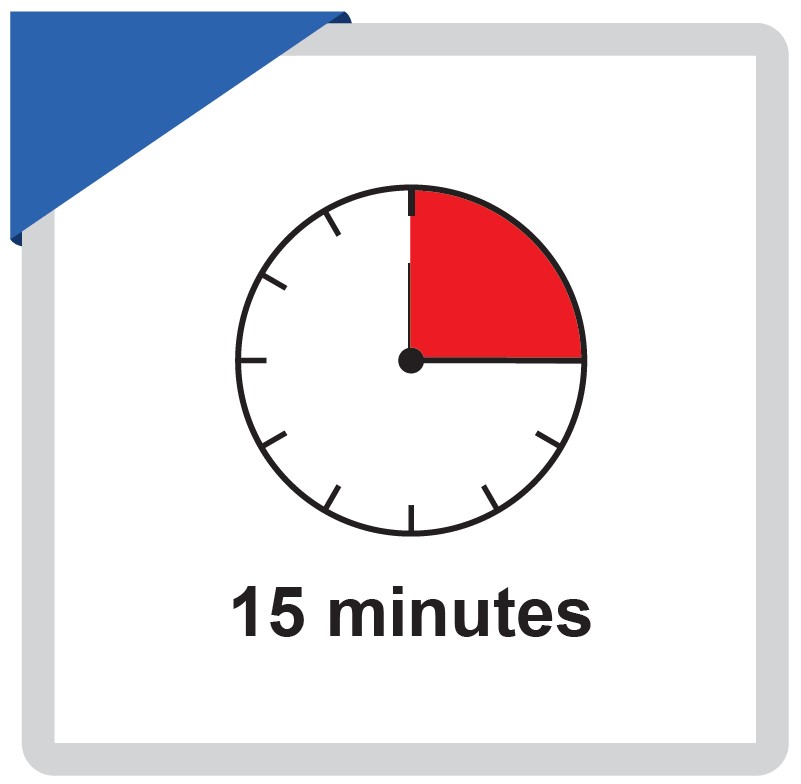
Read result at 15 minutes after adding buffer, do not interpret the results after 30 minutes.
Results Interpretation
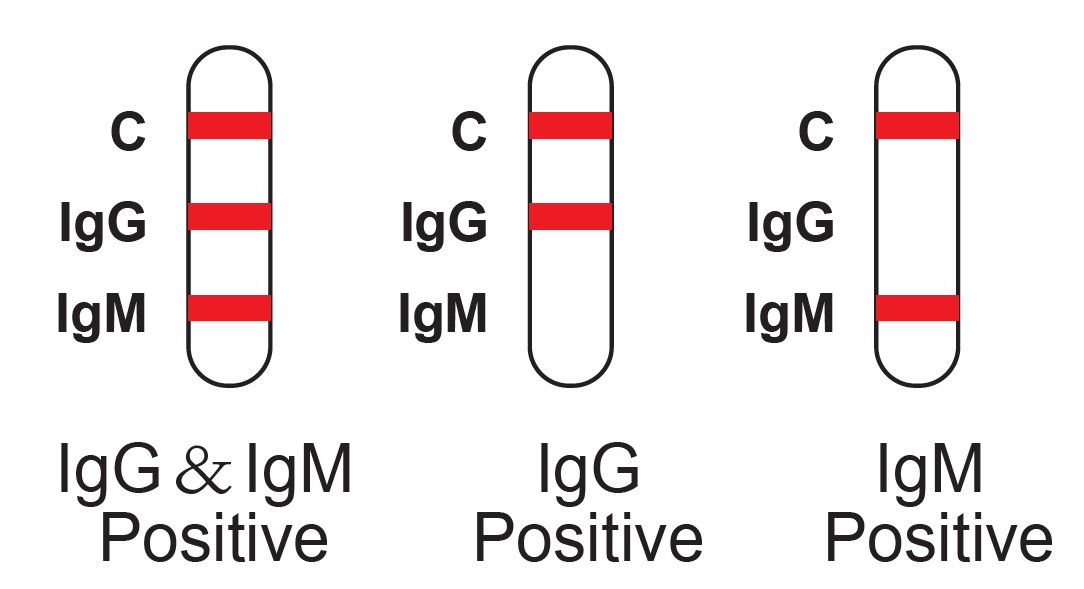
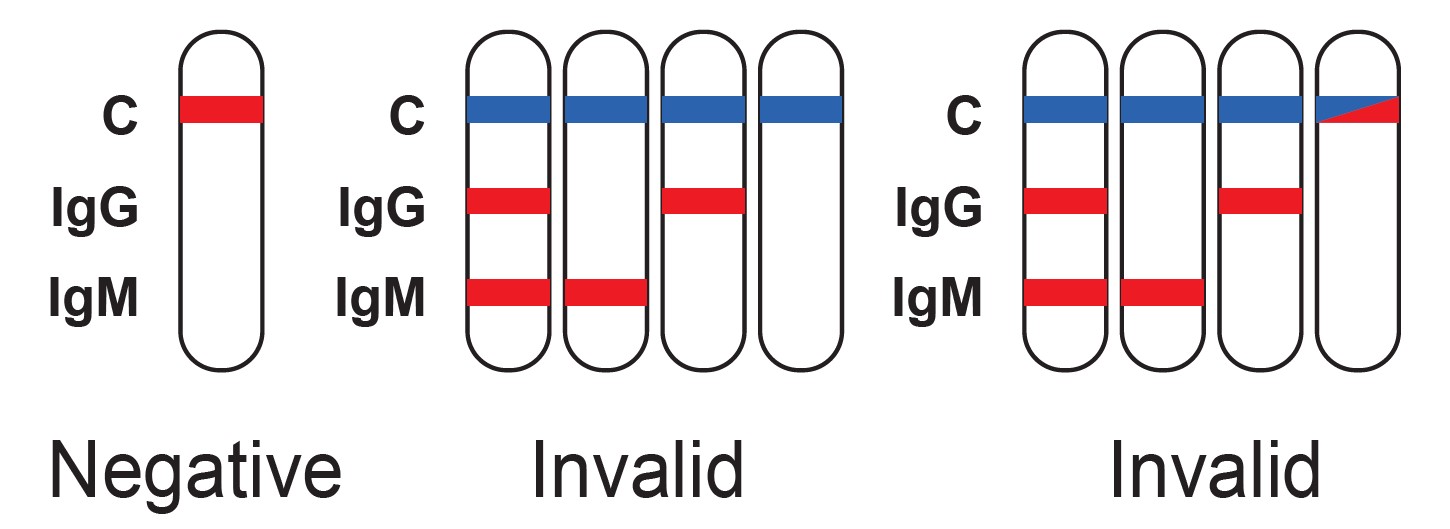
The detection of SARS-CoV-2 IgG/IgM antibodies is dependent upon proper specimen collection, handling, storage, and preparation, per the Instructions for Use (IFU). Failure to observe proper procedures in any one of these steps can lead to incorrect results.
Due to the highly contagious nature of SARS-CoV-2 and the devastating impacts on healthcare systems and the world economy, COVID-19 has been designated as a pandemic by the World Health Organization (WHO). Rapid testing, systematic screening and detection of both clinical and asymptomatic COVID-19 cases are critical components to mitigating the virus’ spread.
